Intermolecular forces include hydrogen bonds, dipole dipole forces, ion-dipole forces, and London dispersion (van der Waal’s) forces. The following notes can help you learn about each of these forces.
Online PDF of notes about IMF:
https://web.gccaz.edu/~kimld88531/chm130lec_files/Ch%2014%20OER.pdf
Practice: For each of the following, predict ALL the intermolecular forces that would exist within a sample of like molecules of each compound.
Example 1: Carbon tetrachloride
Answer 1: This is a nonpolar molecule. (You can draw the Lewis structure and see that this molecule is symmetrical and has tetrahedral geometry, making it nonpolar.) The only force that can exist between nonpolar molecules is London dispersion forces.
Example 2: Acetic Acid CH3COOH
Answer 2: Acetic Acid has the Lewis structure seen below.
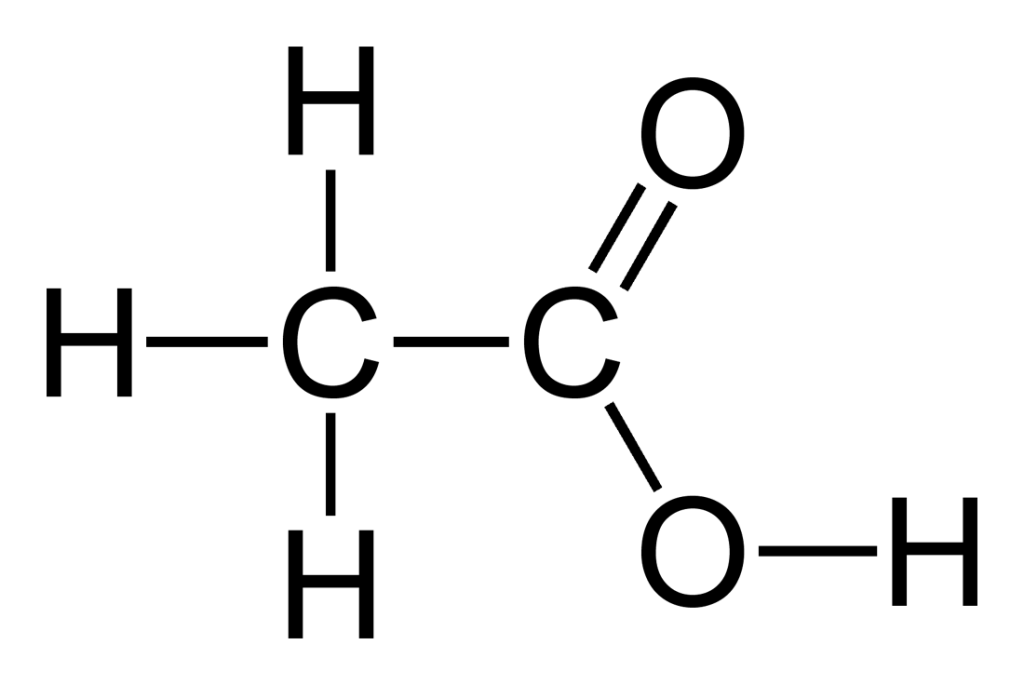
Because of the electronegative oxygen, acetic acid is a polar molecule. (The right hand side of the molecule bears a partial positive charge.) Because the molcule is polar and the oxygen is connected to a hydrogen, this molecule has hydrogen bonds. Hydrogen bonds are a type of dipole dipole force. All molecules (both polar and nonpolar) also have dispersion forces.
Example 3: Acetone CH3COCH3
Answer 3: The Lewis structure for acetone is shown below:
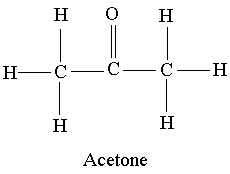
Because of the presence of the doubly bonded oxygen, acetone is a polar molecule. However, this oxygen is attached to a carbon and not to a hydrogen as in the previous example. For this reason, acetone has dipole dipole forces due to its polarity as well as the dispersion forces present in all molecules.
Example 4: Dihydrogen sulfide H2S
Answer 4:The Lewis structure for H2S shows four electron domains (two bonding domains between the H and S atoms plus two nonbonding domains in the form of lone pairs on the sulfur.) This means that the dihydrogen sulfide molecule has a bent geometry and is polar. The molecule has dipole dipole forces due to its polarity as well as the dispersion forces present in all molecules. (Note: This compound does not experience hydrogen bonding, because for a substance to have H bonding, the hydrogen must be connected to O, N, or F.)
Example 5: Rank the following compounds from the one with the weakest intermolecular forces to the one with the strongest intermolecular forces.
HF, F2, SO2, and PCl3
Answer 5: To answer this question, determine the dominant intermolecular force in each compound, then rank the forces from weakest to strongest. The order will be:
Weakest: F2 (nonpolar covalent molecule with only dispersion forces)
Middle: SO2 and PCl3 (polar molecules both with dipole dipole forces)
Strongest: HF (polar molecule with hydrogen bonding)
