Previously in chemistry class, you learned to draw the Lewis and VSEPR structures of molecular compounds. Lewis structures provide information about how atoms are connected in molecules, which, in turn, allows us to predict properties and chemical behavior. You also learned to determine and draw the 3-D geometry (VSEPR shapes) of molecules. You may remember that molecular polarity is based upon the 3-D shape of compounds.
The polarity of a compound provides information about the properties of the compound. Learning about intermolecular forces requires you to apply the molecular geometry and polarity concepts.
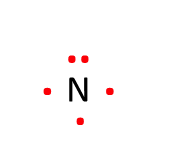
Review: Lewis Dot Symbols
Lewis dot symbols are used to represent the valence electrons around the element.
Recall the steps for drawing Lewis dot symbols:
- Write the symbol for the element
- Determine the number of valence electrons the element has
- Draw the valence electrons as “dots” surrounding the element
- We designate 4 sides to the element symbol: top, right, bottom, left
- Before you can put two dots on the same side, each side must have one dot (2 max)
Review: Lewis Structures
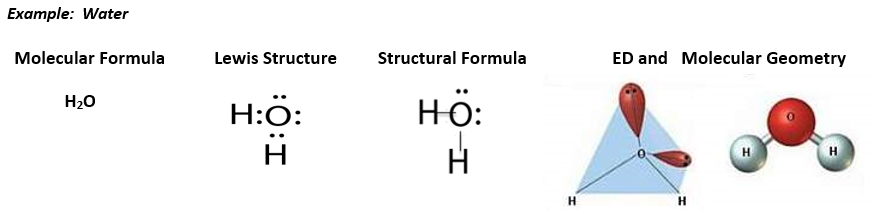
Now that you remember how to draw Lewis dot symbols for individual elements, let’s recall how to draw the Lewis structure for molecular compounds. The Lewis structure sets us up to draw structural formulas, which use straight lines to represent the two shared electrons in a bond. The structural formulas can then be used to draw the 3-D structures (molecular geometry) of covalent compounds from the electron domain (ED) geometry.
The structures of methane, ammonia, water, and hydrogen fluoride are shown below.
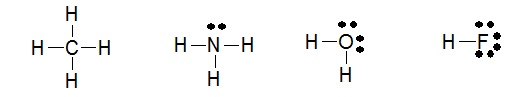
Determine the Central Atom:
- Hydrogen is NEVER central
- Often, the first element in the formula is the central atom. (In PF3, P is central.)
- The lease electronegative atom is central
- If there is more than one carbon in a compound, the carbon atoms are often bonded together.
Single Bond: A covalent bond that involves sharing one pair of electrons. It is represented by a single dash between two atoms

Double Bond: A covalent bond that involves sharing two pairs of electrons.
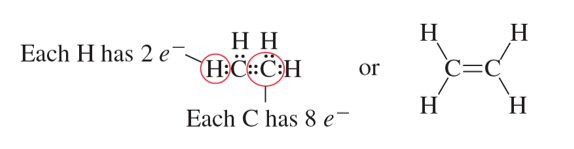
Triple Bond: Covalent bond formed when atoms in the bond share three pairs of electrons
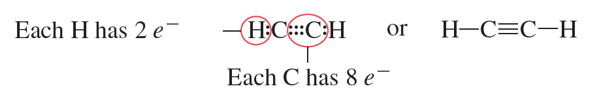
Steps for Drawing a Structural Formula:
- Using the periodic table, count the total number of valence electrons in the molecule.
- Draw the central atom and attach it to the exterior atoms with the correct number of bonds.
- Fill in octets with lone pairs of electrons
- Count the number of electrons in the structure and verify that it equals the total number of valence electrons for the atoms in the molecule.
Formal Charge
Below you see two possible Lewis structures for carbon dioxide. These Lewis structures both follow the octet rule, but which Lewis structure is better?
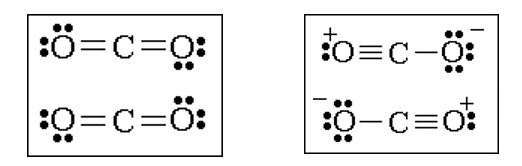
Recall that the purpose of a Lewis structure is to provide a simple model from which predictions about molecular structure could be made. The concept of a formal charge has been found useful for determining the best (most useful) Lewis structure for a molecule. Formal charges are assigned to atoms in molecules according to a set of rules.
Formal charge of an atom = # valence electrons the atom has according to the periodic table – number of assigned electrons in the proposed Lewis structure
Where the number of assigned electrons to a given atom is determined as follows:
- Non-bonding electrons (lone pairs) are assigned to the attached atom.
[THUS, add two for each lone pair on the atom]
- Bonding electrons, i.e. shared electrons are evenly divided between the two bonded atoms.
[THUS, add one for each single bond, two for each double bond and three for a triple bond]
A Lewis structure is not considered complete unless the (non-zero) formal charges are indicated on the structure. As a rule of thumb, the most appropriate Lewis structure will be the one that minimizes formal charges. And formal charges greater than ± 1 are never found in good Lewis structures.
Based on the concept of formal charge, the Lewis structure on the left above is the better Lewis structure for CO2.
When drawing Lewis structures,
- select the structure that has the least formal charge
- place a negative charge (if needed) on the most electronegative atom
Examples
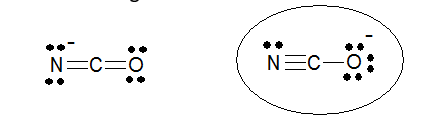
The circled structure for cyanate ion is preferred because the negative charge is on oxygen, the more electronegative atom.
Molecular Geometry: Models in Three Dimensions
Consider the Lewis structure for methane seen below:
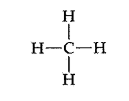
The drawing looks flat and two dimensional on the page, however in three dimensions the hydrogens arranged around the central carbon spread out in order to minimize the electron repulsions in the bonds. The molecule assumes a three dimensional tetrahedral arrangement where the four hydrogens spread out into positions 109.5 degrees apart. It is a symmetrical arrangement in which all four positions are equivalent, thus the molecule is nonpolar. The drawing below is a sketch representing the 3-D structure of the molecule.
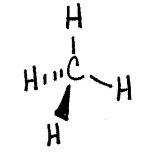
To determine the 3-D electron domain and molecular geometries you can use the handout below:
Molecular Geometry Example:
Let’s look at the Lewis structure for water again:
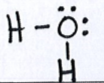
From the Lewis structure we see that water has 4 electon domains around the central atom: 2 bonding domaisn and 2 nonbonding (loan pair) domais. This means that the electron domain geometry for water is tetrahedral and the molecular geometry is bent. Because of the loan pairs on the central atom, water is not a symmetrical molecule and is polar. The oxygen end of the water molecule is partially negative and the hydrogen end of the water molecule is partially positive.
