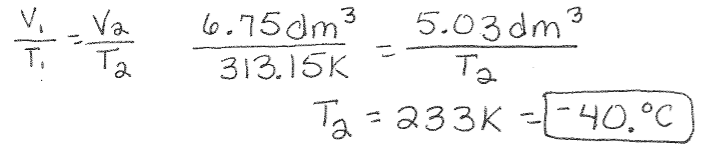From his experiments Jacques Charles proposed that the volume of a fixed quantity of gas at constant pressure is directly proportional to its absolute temperature.
You may have used a pop up turkey thermometer to cook your Thanksgiving turkey. These work because as the temperature goes up while cooking, the air in the thermometer expands and pops the plunger.
Charles’s Law Example: A bubble of carbon dioxide gas in unbaked bread has a volume of 1.15cm3 at a temperature of 22°C. What volume will the bubble have when the bread is baked and the bubble reaches a temperature of 99°C?
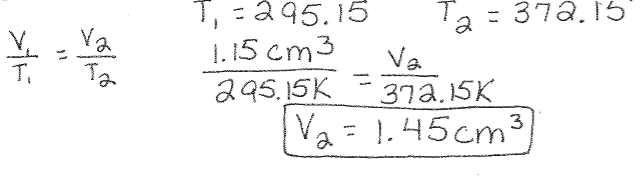
Charles’s Law Example: A perfectly elastic balloon contains 6.75 dm3 of air at a temperature of 40.°C. What is the temperature (in Celsius) if the balloon has a volume of 5.03 dm3?