From his experiments Robert Boyle proposed that the volume of a fixed quantity of gas at constant pressure is inversely proportional to its pressure.
P1V1 = P2V2
The process of breathing is an illustration of Boyle’s Law. As you breath in, your diaphragm moves down, your ribs expand, and the volume of your lungs increases. Boyle’s Law tells us that when volume of the lungs increases, the pressure decreases. When the pressure inside the lungs is less than atmospheric pressure, air is forced into the lungs until the pressures are equal. The opposite happens as you breath out. The diaphragm rises and the ribs contract, causing the volume of the lungs to decrease. This raises the pressure within the lungs and forces air out.
Boyle’s Law Example: A gas cylinder with 0.722m3 of hydrogen gas is at a pressure of 10.6 atm. If the gas is used to fill a balloon at a pressure of 0.96atm, what is the volume in cubic meters of the filled balloon?
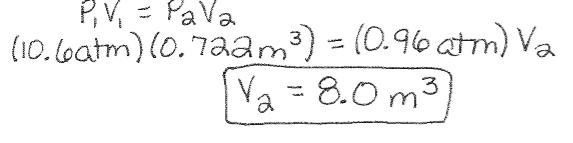
Boyle’s Law Example: A weather balloon has a maximum volume of 7.50 x 103 L. The balloon contains 195L of helium gas at a pressure of 0.993atm. What will be the pressure when the balloon is at its maximum volume?

