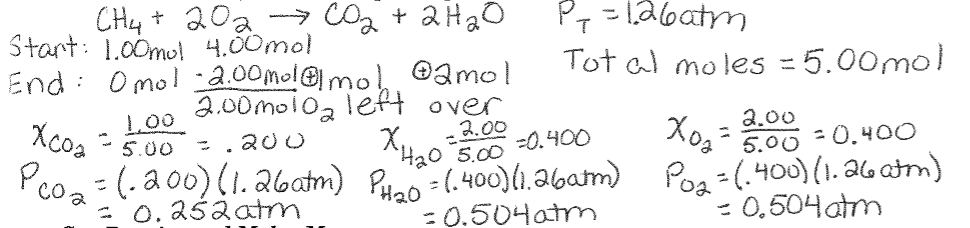The ratio of the partial pressure a single gas contributes and total pressure is equal to the mole fraction (X). (Note: Mole fraction is a dimensionless number.)

Mole Fraction and Partial Pressure Example: When one mole of methane is heated with four moles of oxygen gas, the following reaction occurs:
CH4(g) + 2O2(g) à CO2(g) + 2H2O(g)
Assuming all the methane is converted to carbon dioxide and water, what are the mole fractions of oxygen gas, carbon dioxide, and water in the resulting mixture? If the total pressure of the mixture is 1.26atm, what are the partial pressures?