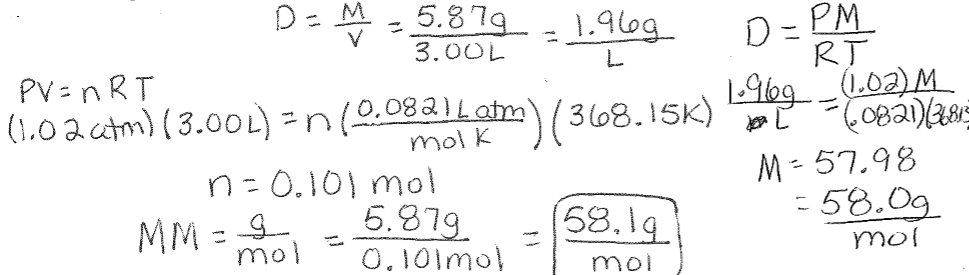Just as solids have density expressed as units of mass over volume, gases also have density.
A rearrangement of the ideal gas law equation gives us the density equation for gases:
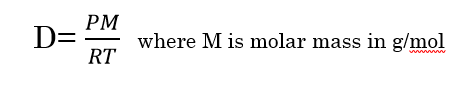
Molar Mass and Density at STP Example: The density of a gaseous compound containing carbon and oxygen is 1.964 g/L at STP. Determine the molar mass of the compound.

Molar Mass and Density Example (Not at STP): Acetone is used in nail polish remover. A sample of liquid acetone is placed in a 3.00L flask and vaporized by heating to 95⁰C at 1.02atm. The vapor filling the flask weighs 5.87g. What is the density of acetone vapor under these conditions? Calculate the molar mass of acetone.