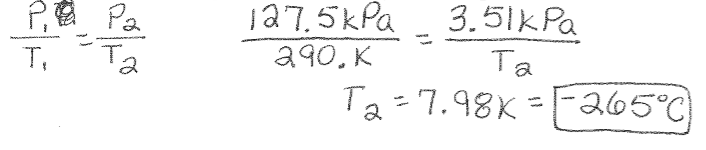From his experiments Joseph Gay-Lussac proposed that if amount of gas and volume are held constant, then pressure and absolute temperature are directly proportional.
=
Making popcorn is an example of Gay-Lussac’s law. As the temperature goes up during heating, the pressure of the water vapor within the kernel goes up until eventually the kernel explodes and “pops.”
Gay-Lussac’s Law Example: The pressure in a car tire is 2.50 atm at a temperature of 33°C. What would the pressure be if the tire is cooled to 0°C?
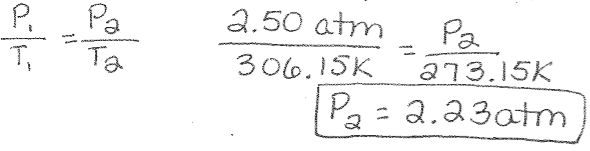
Gay Lussac’s Law Example: A container filled with He gas has a pressure of 127.5kPa at a temperature of 290.K. What is the temperature (in Celsius) when the pressure is 3.51kPa?