Ideal gases follow our assumptions of the kinetic molecular theory. They always obey PV=nRT, assume molecules have zero volume, and assume no molecular attractions or repulsions. However, real gases can show some deviation from ideal behavior. They obey PV=nRT only at very low pressure and high temperature, have a small (but nonzero) volume, and have small molecular attractions or repulsions.

For real gases at high pressures, atoms are more densely packed and take up a more significant portion of the gas volume. For real gases at low temperatures, gas atoms spend more time interacting with each other and less time colliding with the walls, thus reducing the pressure. A scientist named Van Der Waal’s created an equation which corrects for these deviations of volume and pressure experienced by real gases:
The van der Waals Equation models the behavior of non-ideal (real) gases.
Effect of Finite Volume Vreal = Videal – b
b is an experimentally determined constant
This part of the Van der Waals equation corrects for volume.
Effect of Intermolecular Attraction:
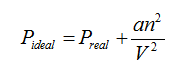
This part of the Van der Waal’s equation corrects for pressure.
van der Waal’s Equation for Non-ideal Gases
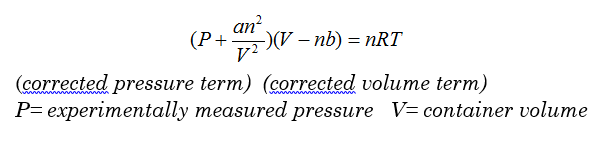
Van der Waal’s Example: According to the equation, is the volume for a real gas more or less than the volume of an ideal gas? According to the equation, is the pressure for a real gas more or less than the pressure of an ideal gas?
Answer: Real volume is less than the total volume of the container, so volume of real gases is less than the volume of ideal gases. Therefore, measured pressure would be less than ideal pressure.
