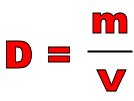Density is an important property for determining the identity of a sample of matter. It is defined as the ratio of mass to volume.
Remember that density is an intensive physical property. Since it is a ratio, changing the size of the sample and volume of the sample will not change the ratio. For this reason, each element has its own unique density which can be used to identify that element.
It is a combination of several of the fundamental units so it is a derived unit. Two units are combined—a mass unit and a volume unit. The density of solids and liquids is usually expressed as g/ cm3 and the density of gases as g/ L; however, other units of mass or volume may also be used.
Density Practice Problems:
- An object has a volume of 825 cm3 and a density of 13.6 g/cm3. Find its mass.
- What is the density of a gas when 14.28 g takes up 30.0 L of space?
- What is the mass of an object taking up 75 cm3 of space and having a density of 2.2 g/cm3?
- Gold has a density of 19.3 g/cm3. How much volume would 7.00 g of gold occupy?
- A 44.00 g block has a measured volume of 35 cm3. What is its density?
- 15.95 g of a substance has a density of 7.000 g/m3. How much space does it occupy?
- Oxygen gas has a density of 1.33 g/L under normal conditions. What is the mass of 20.00 L of oxygen gas?
- A liquid has a density of 0.87 g/mL. What volume is occupied by 25g of the liquid?
- An icicle is placed in 55.2 mL of ethanol so that it is completely submerged. The volume of the ethanol in the container increases to 70.6 mL before the icicle begins to melt. What is the mass of the icicle? (Use the density of ice = 0.915 g/cm3.)
- What is the mass of a cube of aluminum that is 3.0cm on each edge? The density of aluminum is 2.7g/cm3.
More Challenging Problems:
- You are given a bottle that contains 4.59 cm3 of a metallic solid. The total mass of the bottle and the solid is 35.66g. The empty bottle weighs 14.23g. What is the density of the solid?
- The earth has a mass of approximately 5.99 x 1024 kg and a radius of 6.38 x 106 meters. (Use the formula for volume of a sphere to calculate its volume) Find the density of the earth in g/cm3
- A graduated cylinder was filled with water to the 15.0 mL mark. When placed on the balance the cylinder and water together had a mass of 27.35g. An object made of silver was placed in the water and became completely submerged. The water level rose to 18.3 mL. When reweighed, the cylinder, water, and silver had a mass of 62.00g. Calculate the density of the silver.
Check your answers to the density practice problems here.