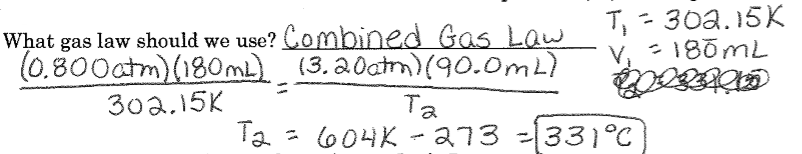Mixed Gas Laws Example #1: If I initially have a gas with a pressure of 84 kPa and a temperature of 350 C and I heat it an additional 230 degrees celsius, what will the new pressure be? Assume the volume of the container is constant.

Mixed Gas Laws Example #2: The air in a piston occupies 24.8 cm^3 at 1.12 atm. The piston is compressed to increase the pressure to 2.64 atm. Assuming constant temperature, what is the new volume in L?
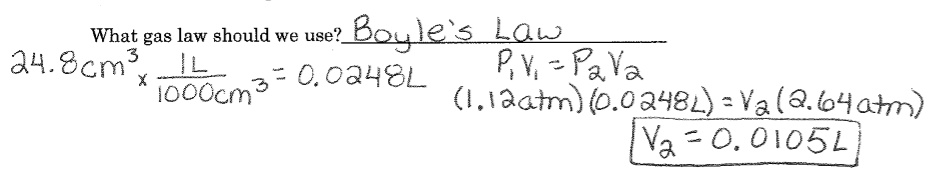
Mixed Gas Laws Example #3: 4.40 L of a gas is collected at 50.0 °C. What will be its volume upon cooling to 25.0 °C?

Mixed Gas Laws Example #4: A sample of helium gas has a volume of 0.180 L, a pressure of 0.800 atm and a temperature of 29°C. What is the new temperature(°C) of the gas at a volume of 90.0 mL and a pressure of 3.20 atm?