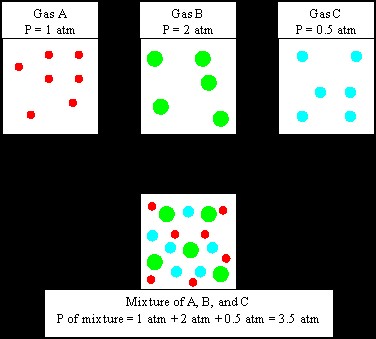
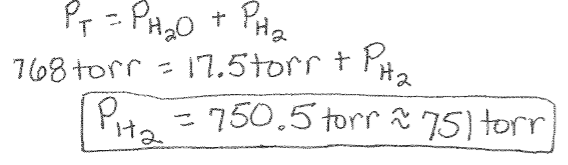If gases are mixed in a container, we can assume that the gas molecules will be far apart
and behave independently. The partial pressure is the pressure exerted by one particular
component of a gas mixture. The total pressure of all gases in a container is equal to the sum of
the pressures that each gas would exert if it were present alone. This observation is called
Dalton’s Law:
Ptotal= P1 +P2+ P3+ …
Dalton’s Law of Partial Pressures is particularly useful when a gas is collected “over water.” Since gases mix readily, they must be collected in an environment where mixing cannot occur. This can be done under water because water displaces the air. When we say that a gas is collected “over water” we mean that a container is filled with water and the gas is bubbled through the water and into the container. Therefore, the pressure inside the container is from the gas AND the water vapor. The vapor pressure of water at various temperatures is a constant which can easily be looked up in reference tables.
Ptotal= Pgas + Pwater
Dalton’s Law Example of Collecting a Gas Over Water: A student collects some hydrogen gas over water at 20°C and 768 torr. What is the pressure of hydrogen gas? (The vapor pressure of water at this temperature is 17.5 torr.)