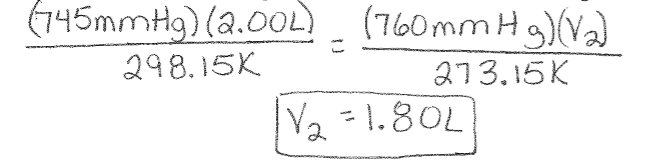The experimental observations of the individual gas laws can be united in the combined gas law.
Combined Gas Law Example: A scientist has a sample of gas that was collected several days earlier. The sample has a volume of 392 cm3 at a pressure of 0.987atm and temperature of 21°C. On the day the gas was collected the temperature was 13°C and pressure was 0.992 atm. What volume did the gas have on the day it was collected?
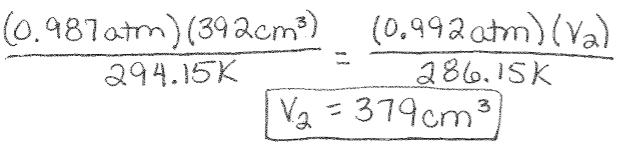
Combined Gas Law Example: 2.00L of a gas is collected at 25°C and 745mmHg. What is the volume at STP?