Gay Lussac had seen in experiments that at a given temperature and pressure the volumes of gases that react with one another are ratios of small whole numbers. He called this the law of combining volumes. Based on this, Avogadro hypothesized that equal volumes of gases at the same temperature and pressure contain the same number of molecules. He proposed Avogadro’s law that the volume of gas at a given temperature and pressure is directly proportional to the number of moles of gas. For gases at STP, the volume occupied by 1 mol of any as is 22.4L.
Avogadro’s Law Example: What is the volume of 4.15 x 10^22 molecules of a gas at STP?
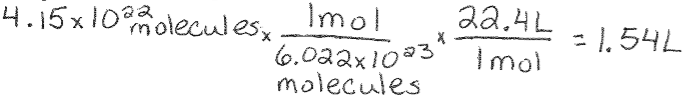
Avogadro’s Law Example: What is the volume of 8.25g sulfur dioxide gas at STP?

Avogadro’s Law Example Using Stoichiometry: If 1.1 g hydrogen peroxide is decomposed, what is the volume of oxygen gas at STP? 2H2O2 (l) à 2H2O (g) + O2(g)
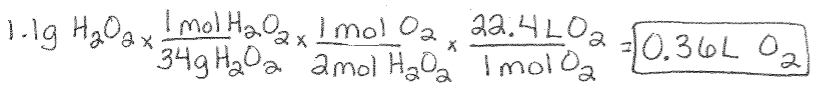
Avogadro’s Law Example Using Stoichiometry: Potassium chlorate decomposes to produce potassium chloride and oxygen gas. How many grams of potassium chlorate are required to produce 9.00L of oxygen gas at STP?
2KClO3 à 2KCl + 3O2

